The Food and Drug Administration (FDA) has recently started issuing “pre-notices” for potential non-compliance with rules for registering clinical trials and reporting results to ClinicalTrials.gov. These pre-notices are meant to warn parties that they could face eventual civil penalties for failing to publicly register and report results (including demographics) from clinical trials. Since January 2017, registration of trials and reporting of results for many pharmaceutical clinical trials has been mandatory under the Final Rule of the Food and Drug Administration Amendments Act (FDAA) of 2007. Our 2023 Demographic Reporting and Mix in Clinical Trials report shows that while results reporting has improved over time, the reporting rate for demographic information remains low, particularly for non-drug trials, which are not covered by the FDAA.
The day after the Final Rule of the FDAA went into effect, the National Institutes of Health (NIH) made a similar policy change: just as the FDAA required registration and reporting for drug trials, the NIH started requiring registration and reporting for any clinical trials receiving NIH funding. This included not only trials of pharmaceuticals, but any clinical trial, including, for example, studies of surgeries, diagnostics, or behavioral interventions.
The NIH is the largest single public funder of clinical research in the US, and the desire to receive repeat funding provides a powerful incentive for those running trials to collect the required information (which includes study participant demographics) and publicly report the results to ClinicalTrials.gov. The impact of the policy change by the NIH can be easily seen in the Aggregate Analysis of ClinicalTrials.gov (AACT) database. Following the near-simultaneous enactment of the FDAA Final Rule and the NIH mandatory reporting requirement, reporting of demographic results improved across all studies but increased much more rapidly for studies funded by the NIH.
For example, 14.36 percent of clinical trials that finished in the 3 years before the FDAA Final Rule and the NIH mandatory reporting requirement reported demographic information on the ethnicity of study participants. This overall rate of reporting information on ethnicity increased to 23.9 percent for the 3 years following the policy changes, a 66.9 percent increase in reporting. However, studies funded by the NIH went from 23.6 percent reporting to 52.1 percent reporting, an increase of over 120 percent (a more than doubling of the reporting rate). This outperformed the improvements in reporting rates for other large funder classes including industry (funders such as pharmaceutical or device companies) and universities and hospitals (the largest group of funders, funding just under 70 percent of all clinical trials). There was a similar (but slightly less pronounced) pattern for reporting of racial demographics: the average clinical trial reporting rate for racial demographics improved by 64.4 percent, whereas the reporting rate for studies funded by the NIH improved by 88.0 percent.
We would not expect to see 100 percent reporting, as some types of trials can delay reporting (for example, a product not yet on the market can delay reporting results to protect proprietary information ahead of release). However, the differential trends in reporting tell a clear story: mandatory reporting rules have greatly improved reporting rates; and the broad rules used by the NIH have been the most effective.
Representation in trials is a non-trivial concern, as multiple studies have documented meaningful differences in treatment effectiveness based on demographics. To assess whether there is adequate representation in studies, which is needed to understand if a given intervention has relevant differences in impact based on demographics, demographic information needs to be collected and reported in the first place. A straightforward way to improve data quality across the board is to have other funders of research follow the NIH’s lead: require reporting of demographic information for all funded clinical trials.
Reporting Rate of Ethnicity Demographics to ClinicalTrials.gov for Trials with Different Funders
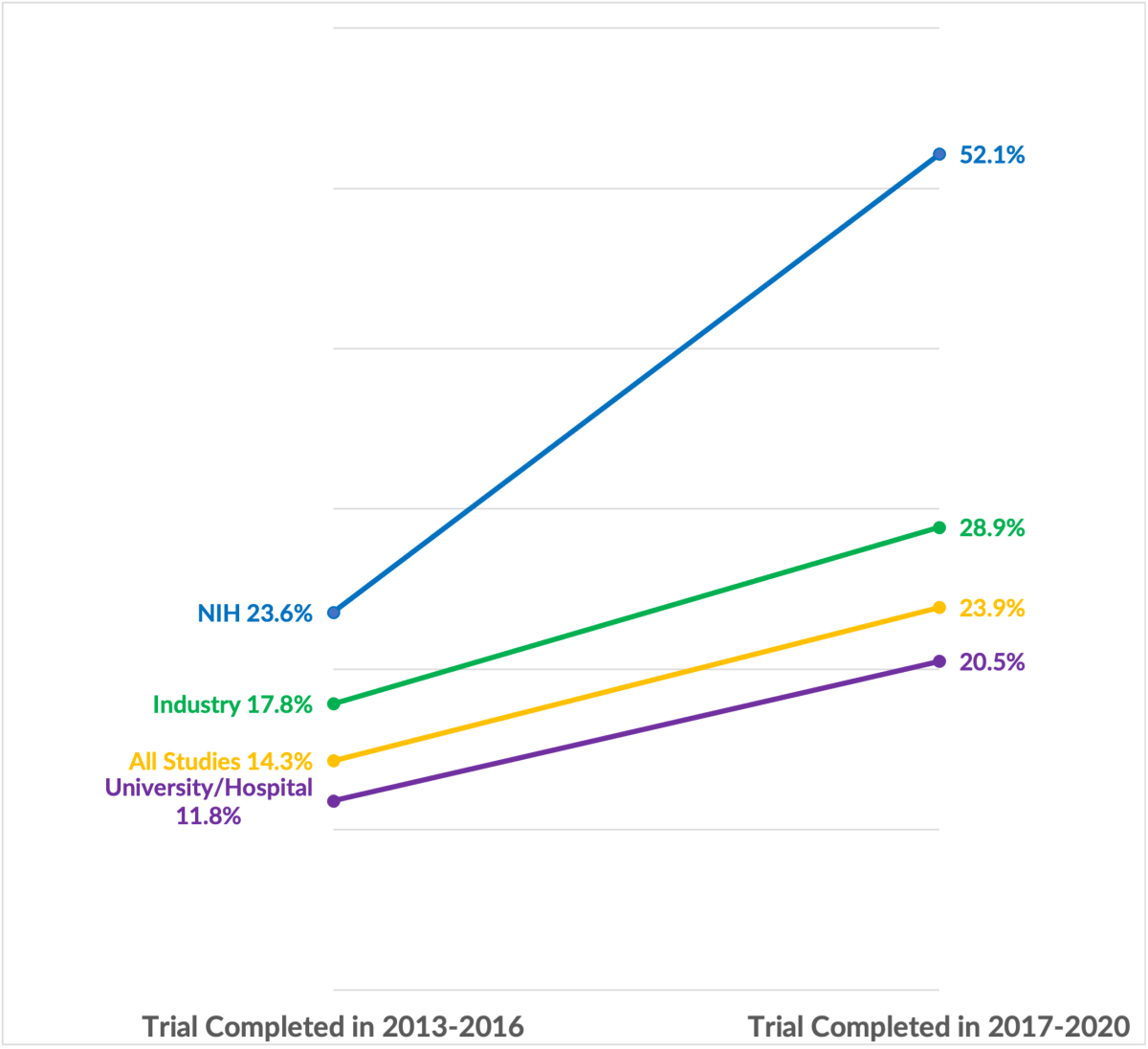
Source: Author’s analysis using the AACT (2024)